Section: New Results
Proliferation dynamics and its control
Proliferation dynamics in cell populations
Participants : José Luis Avila Alonso [DISCO project-team, Inria Saclay IdF] , Annabelle Ballesta, Gregory Batt [CONTRAINTES project-team] , François Bertaux, Frédérique Billy, Frédéric Bonnans [Commands project-team, Inria Saclay IdF] , Catherine Bonnet [DISCO project-team, Inria Saclay IdF] , Jean Clairambault, Marie Doumic, Xavier Dupuis [Commands project-team] , Ján Eliaš, Germain Gillet [IBCP, Université Cl. Bernard Lyon 1] , Pierre Hirsch [INSERM Paris (Team18 of UMR 872) Cordeliers Research Centre and St. Antoine Hospital, Paris] , Pierre Magal [University Bordeaux II] , Anna Marciniak-Czochra [Institute of Applied Mathematics, Universität Heidelberg] , Jean-Pierre Marie [INSERM Paris (Team18 of UMR 872) Cordeliers Research Centre and St. Antoine Hospital, Paris] , Roberto Natalini [IAC-CNR, Università Sapienza, Rome] , Silviu Niculescu [DISCO project-team, Inria Saclay IdF] , Hitay Özbay [Bilkent University, Ankara, Turkey] , Benoît Perthame, Szymon Stoma [CONTRAINTES project-team] , Ruoping Tang [INSERM Paris (Team18 of UMR 872) Cordeliers Research Centre and St. Antoine Hospital, Paris] , Vitaly Volpert [CNRS Lyon, UMR5208, Camille Jordan Institute, Lyon] , Jorge Zubelli [IMPA, Rio de Janeiro] .
-
Transition kernels in a McKendrick model of the cell division cycle. This theme, after a rich harvest of publications (most of them in 2013 and even 2014), is awaiting new developments, since of the main two young researchers on this theme, F. Billy has concluded her 2-year Inria postdoc at Bang, leaving for an industrial company in November 2012, while O. Fercoq (team MaxPlus, Saclay) has defended his PhD thesis at École Polytechnique in September 2012, only to leave for a postdoc position dedicated to optimisation theory in Edinburgh.
-
Modelling haematopoiesis with applications to AML. This theme has been active through a collaboration with Inria teams Commands (F. Bonnans, X. Dupuis) and Disco (J.L. Avila Alonso, C. Bonnet, Hitay Özbay, S. Niculescu), and J.-P. Marie's team at St Antoine Hospital leukaemic tumour bank, where A. Ballesta, Cancéropole IdF-Inria postdoc has been detached (ending in January 2013) to identify parameters of a model of acute myeloblastic leukaemia (AML) in patient fresh cell cultures with and without anticancer drugs. This work has led to several presentations, and publications are in preparation. In a book chapter summing up the PhD work of J.L. Avila Alonso [26] , and in two submitted conference papers [28] , [29] , a new model of haematopoiesis for AML is presented, including phases of the cell division cycle and maturation stages, with targets for therapeutic control.
-
Hybrid models. Systems combining PDEs and discrete representations in hybrid models, with applications to cancer growth and therapy, in particular for AML, are the object of study of the ANR program Bimod, coordinated by V. Volpert (Lyon), associating CNRS (V. Volpert, Lyon), Bordeaux II University (P. Magal) and the Bang project-team.
-
With G. Gilllet (professor at IBCP/Lyon), A. Ballesta and M. Doumic have designed a mathematical ODE model for the mitochondrial pathway of apoptosis, focused on the early phase of apoptosis (before the cytochrome C release). This model has been validated by experimental data carried out in G. Gillet's lab and applied to propose new therapeutic strategies against cancer [6] .
-
Molecular model of the activity of the p53 protein. This work, firstly the object of Luna Dimitrio's PhD thesis [37] , who left in 2012 for the pharmaceutic industry (SANOFI), has been continued since a new PhD student, Ján Eliaš, has taken over this theme in September 2012 in a new PhD thesis at UPMC, under the supervision of J. Clairambault and B. Perthame. His work has given rise in 2013 to 2 publications [14] , [32] .
-
TRAIL - induced apoptosis in HELA cells Explaining cell-to-cell variability is a major step towards understanding how cancer cells escape action of chemotherapeutic drugs. We set up and studied an integrated model of stochastic gene expression, deterministic translation and protein degradation capable of explaining fractional killing and reversible resistance in Hela cells in response to treatment with TNF-Related Apoptosis Inducing Ligand, TRAIL (Bertaux, Stoma, Drasdo, and Batt, submitted). The results of the model suggests that stochastic fluctuations are a fundamental determinant in understanding cell-to-cell variability, and identified relations between the characteristic time scales of the processes at which stochasticity should play a particular important role.
Physiological and pharmacological control of cell proliferation
Participants : Annabelle Ballesta, Frédérique Billy, Jean Clairambault, Sandrine Dulong [INSERM Villejuif (U 776)] , Olivier Fercoq [MaxPlus project-team] , Stéphane Gaubert [MaxPlus project-team] , Thomas Lepoutre [Dracula project-team] , Francis Lévi [INSERM Villejuif (U 776)] .
-
Periodic (circadian) control of cell proliferation in a theoretical model of the McKendrick type. This theme (cf. supra “transition kernels...”) has been continued [9] , [27] , [7] , [8] , [31] . Whereas transition kernels between cell cycle phases without control have been experimentally identified in cell cultures by FUCCI imaging [9] , their circadian control remains elusive and has been modelled on the basis of gating by plain cosines representing the influence exerted on these transition kernels by circadian clocks. To go further, it would be necessary to have access by cell imaging to the activity of the best physiological candidates to such gating, namely the cyclin-Cdk complexes, together with the activities of the clock-controlled proteins Wee1 and p21, which thus far have remained unavailable to us through biological experimentation with imaging. A 12-year collaboration work with Francis Lévi on (circadian) chronotherapeutic optimisation in cancer is reported in [30] .
-
Intracellular pharmacokinetic-pharmacodynamic (PK-PD) models for anticancer drugs. This theme has continued to be developed with new publications for the drugs irinotecan [5] , 5-fluorouracil and oxaliplatin [31] , and with a recent mini-review by A. Ballesta and J. Clairambault on mathematical models of treatment of metastatic colorectal cancer [4] .
Optimisation of cancer chemotherapy and cancer radiotherapy
Participants : Juan Carlos Alfonso [University Complutense, Madrid, Spain] , Annabelle Ballesta, Frédérique Billy, Frédéric Bonnans [Commands project-team] , Rebecca Chisholm, Jean Clairambault, Sandrine Dulong [INSERM Villejuif (U 776)] , Xavier Dupuis [Commands project-team] , Alexandre Escargueil [INSERM and UPMC, St Antoine Hospital] , Olivier Fercoq [MaxPlus project-team] , Stéphane Gaubert [MaxPlus project-team] , Miguel Angel Herrero [University Complutense, Madrid, Spain] , Michael Hochberg [ISEM, CNRS, Montpellier] , Dirk Drasdo, Nick Jagiella, Francis Lévi [INSERM U 776, Villejuif] , Thomas Lepoutre [Dracula project-team] , Tommaso Lorenzi, Alexander Lorz, Luis Núñez [University Complutense, Madrid, Spain] , Benoît Perthame, Emmanuel Trélat [LJLL, UPMC] .
-
Limiting unwanted toxic side effects: age-structured models of the cell cycle. Optimising cancer chemotherapy, in particular chronotherapy, is the final aim of these activities. A classical numerical method of optimization under the constraint of limiting toxicity to healthy tissues has been applied to the McKendrick model of the cell cycle divided in phases, endowed with physiologically based targets for both internal (circadian) and external (pharmacological) control. This model has been partly biologically identified on continuous FUCCI recordings of proliferating NIH3T3 cells in culture media; these data were made available to us within the C5Sys consortium, an ERASYSBIO+ European project. Then additional theoretical characteristics establishing hypothetical differences between healthy and cancer cell populations, relying on different responses to physiological circadian clock influences on gating by Cyclin-Cdk complexes between cell cycle phases, have been used to solve the optimization problem, proposing an optimal drug infusion regimen [7] , [8] , [27] , [9] . Using an even more complex McKendrick-like model of the cell cycle, a connection with previously established PK-PD ODE models of the anticancer drugs 5-Fluorouracil and Oxaliplatin has been established, proposing optimized combined drug delivery flows to solve the same optimization problem [31] .
-
Limiting drug resistance in cancer cell populations: cell Darwinism. This theoretical activity has been continued also in more general settings taking into account another major issue of anticancer treatment, namely resistance to drugs in cancer cells. To this latter aim, we have developed another type of models based on integro-differential equations, which are inspired from those used in ecology for Darwinian evolution [22] . These are aimed at studying another major issue in cancer therapy: appearance of resistances to treatment in tumour cell populations. Indeed, these cell populations, because of their heterogeneity and genomic instability, present an ability to adapt and evolve (in the Darwinian sense) that is much higher than in healthy cell populations [7] , [18] , [35] . The time scales under investigation, much shorter than in ecology, are however much longer than in microbiology, and are those of clinical treatments. Theoretical optimization of external controls representing combined cytotoxic and cytostatic treatments on these models with the aim to limit the emergence of drug resistance are presently under assessment, in collaboration with Emmanuel Trélat (LJLL, UPMC), paper in preparation.
-
Molecular aspects: ABC transporters. From a molecular point of view, studying drug resistance leads to the study of ABC transporters, which is one of the tracks followed by A. Ballesta, following her PhD thesis, in collaboration with F. Lévi's INSERM team in Villejuif [4] , [5] .
-
Optimisation of cell kill in AML. Underway is also the use of methods of optimal control methods developed by the Commands project-team (Frédéric Bonnans, Xavier Dupuis) to optimise therapies in the treatment of Acute Myeloblastic Leukaemia (AML). X. Dupuis has lately produced a paper [40] , accepted for publication in Math. Mod. Phys. Phenom, on optimisation of a combined treatment using a cytotoxic drug (representing Aracytin) and a cytostatic drug (representing AC220, an antagonist of Flt-3 receptors). This work is led in conjunction with the DISCO team, cf. supra “Modelling haematopoiesis with applications to AML”).
-
Estimating dose painting effects in radiotherapy: a mathematical model. Tumor heterogeneity is widely considered to be a determinant factor in tumor progression and in particular in its recurrence after therapy. Unfortunately, current medical techniques are unable to deduce clinically relevant information about tumor heterogeneity by means of non-invasive methods. As a consequence, when radiotherapy is used as a treatment of choice, radiation dosimetries are prescribed under the assumption that the malignancy targeted is of a homogeneous nature. In this work we discuss the possible effects of different radiation dose distributions on heterogeneous tumors by means of an individual cell-based model. To that end, a case is considered where two tumor cell phenotypes are present, which strongly differ in their respective cell cycle duration and radiosensitivity properties. We show herein that, as a consequence of such differences, the spatial distribution of such phenotypes, as the resulting tumor heterogeneity, can be predicted as growth proceeds. As a consequence, heterogeneous dosimetries can be selected to enhance tumor control by boosting radiation in the region occupied by the more radioresistant tumor cell phenotype. It is also shown that, when compared with homogeneous dose distributions as those being currently delivered in clinical practice, such heterogeneous radiation dosimetries fare always better than their homogeneous counterparts (Alfonso et. al., PLoS One accepted [3] ).
Protein polymerisation and application to amyloid diseases
Participants : Annabelle Ballesta, Vincent Calvez [ENS Lyon] , Marie Doumic, Pierre Gabriel, Hadjer Wafaâ Haffaf, Benoît Perthame, Stéphanie Prigent [BPCP, INRA Jouy-en-Josas] , Human Rezaei [BPCP, INRA Jouy-en-Josas] , Léon Matar Tine [SIMPAF project-team, Inria Lille Nord-Europe] .
Published in PLoS One in collaboration with the team of biologists led by H. Rezaei [44] , a new and very complete PDE model for protein polymerisation has been designed. Following F. Charles's work, A. Ballesta has applied this model to Huntington's disease (PolyQ expansion) and compared it with its ODE counterpart, leading to a better understanding of the leading mechanisms responsible for PolyQ fibrillisation. New applications of this framework model are in progress with H.W. Haffaf and S. Prigent.
The eigenvalue problem playing a major role in the representation of Prion proliferation dynamics and, in a more general way, of many fragmentation-coalescence phenomena, the article [36] investigated the dependency of the principal eigenvector and eigenvalue upon its parameters. We exhibited possible nonmonotonic dependency on the parameters, opposite to what would have been conjectured on the basis of some simple cases.
Inverse problem in growth-fragmentation equations
Participants : Marie Doumic, Marc Hoffmann [ENSAE] , Nathalie Krell [Univ. Rennes I] , Patricia Reynaud [CNRS, Nice Univ.] , Lydia Robert [UPMC] , Vincent Rivoirard [Paris IX Univ.] , Léon Matar Tine [SIMPAF project-team, Inria Lille Nord-Europe] .
In collaboration with statisticians (M. Hoffman, Professor at Université de Marne-la-Vallée, V. Rivoirard, MC at Université d'Orsay, and P. Reynaud, CR CNRS at Université de Nice), in the article [38] published in SIAM Num. Anal., we explored a statistical viewpoint on the cell division problem. In contrast to a deterministic inverse problem approach, we take the perspective of statistical inference. By estimating statistically each term of the eigenvalue problem and by suitably inverting a certain linear operator, we are able to construct an estimator of the division rate that achieves the same optimal error bound as in related deterministic inverse problems. Our procedure relies on kernel methods with automatic bandwidth selection. It is inspired by model selection and recent results of Goldenschluger and Lepski.
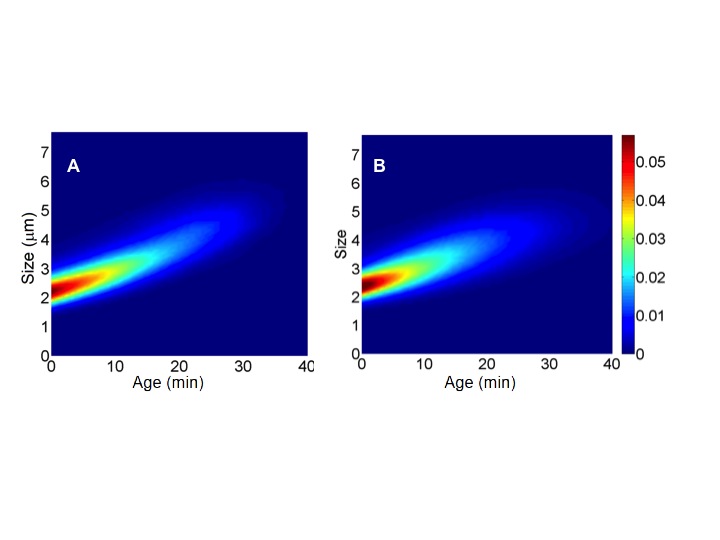
An extension of this work, which consists of the statistical estimation of a branching process modelling the same growth and fragmentation dynamics, has been submitted in [12] , in collaboration with N. Krell, M. Hoffmann and L. Robert. Such methods are indeed successfully applied to investigate bacterial growth, in collaboration with L. Robert (INRA and UPMC), see Figure 1 .
|
In [13] , we generalised the inverse techniques proposed previously in [39] , [43] , in order to adapt them to general fragmentation kernels and growth speeds. The potential applications of this problem are numerous, ranging from polymerisation processes to the cell division cycle. An extension of this work, using refined estimates the Mellin transform of the equation, has just been accepted for publication in Inverse Problems [10] .